2. Carbon dioxide \(\left( \ce{CO_2} \right)\) is a linear molecule. Hence, dipole moment defines as the product of electric charge and distance between the positive and negative species found in the molecule. Hydrogen is less electronegative than nitrogen, but it can also not be chosen as the central atom becausea hydrogen (H) atom can accommodate only 2 electrons; hence it can form a bond with a single adjacent atom only. For O-H bond;The electronegativity difference (EN) = 3.44 2.2 = 1.24This value lies between 0.4 to 2.0, which indicates that the bond between Oxygen (O) and Hydrogen (H) is polar.Hence, the O-H bond is a polar covalent bond. The electronegativity values are equal or nearly equal. Unlike polar bonds, non-polar bonds share electrons equally. The main question in students' minds is if water is polar or nonpolar in chemistry? The arrows are of different lengths, and the arrangement is asymmetrical or uneven. We already discussed that the molecular geometry is bent and electronic geometry is trigonal planar. Pauling also contributed to many other fields besides chemistry. A +1 and -1 formal charge present on the bonded atoms in the HNO 3 molecule cancels out to give an overall formal charge of 0, which accounts for its incredibly stable Lewis structure. As can be seen from the HCN Lewis structure, the electronegativity difference between nitrogen(3.04) and hydrogen(2.2) makes it a polar molecule. From this, you can easily get the idea that the HNO3 molecule is a polar molecule. To determine the polarity of the NO3 ion, we must first account for its properties. While there may be unequal sharing of electrons in the individual bonds, in a nonpolar molecule like HCN these bonds are evenly distributed and cancel out. Waters polarity additionally permits it to take part in an exceptional sort of intermolecular property of creating bonds called hydrogen holding. It interacts with polar solvents such as water due to this charge. The absolute values of the electronegativity differences between the atoms in the bonds H-H, H-Cl, and Na-Cl are 0 (nonpolar), 0.9 (polar covalent), and 2.1 (ionic), respectively. Water, for instance, is a polar solvent that dissolves salts and other polar compounds, but not non-polar substances such as oil. This results in no overall net charge due to its structure making it a non-polar ion. As you know that octet rule required the highest multiple of eight electrons on the total number of valance electrons. So, C2H4 C 2 H 4 is non-polar in nature. So the polarity in this direction and the cancer house So it's not full up May, if you react to be a three to make behind BF three to minus. Bond angle can be defined as the angle formed between central atoms with the two bonded atoms. 3. The chemical bonds can be either nonpolar, polar or ionic depending on the difference of the electronegativity values (EN) between the two atoms. No, CO2 is not polar, even though the bonds are polar. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Figure \(\PageIndex{1}\) shows the electronegativity values of the elements as proposed by one of the most famous chemists of the twentieth century: Linus Pauling. As we already identified, the hydrogen and oxygen atoms are the outer atoms in the Lewis dot structure of HNO3. The more electronegative oxygen atom (O) has a tendency to pull the shared electron pair towards itself, which results in partial positive charge on the hydrogen atom (H) and partial negative charge on oxygen atom (O). It consists of three C-H, one O-H, and one C-O bond. Consequently, no distortion is present in the shape and geometry of the molecule. Using the electronegativity values in Figure \(\PageIndex{3}\), arrange the bonds in order of increasing polarity and designate the positive and negative atoms using the symbols + and .  There are a total of 7 lone pairs in the Lewis structure of HNO3. For O-H bond;The electronegativity difference (EN) = 3.44 2.2 = 1.24This value lies between 0.4 to 1.7, which indicates that the bond between Oxygen (O) and Hydrogen (H) is polar.Hence, the O-H bond is a polar covalent bond. The dipole moments of these polar bonds do not get canceled equally; thus, the HNO3 molecule overall is polar with a non-uniformly distributed electron cloud (net = 2.17 Debye). Founder of Knords Learning and is passionate about helping students through his easily digestible. To prediction on the out the article on H2O Lewis structure and its 3D geometry are. What is the best estimate of the capacity of a juice box? (Wikipedia), A molecule may be nonpolar either when there is an equal sharing of electrons between the two atoms of a diatomic molecule or because of the symmetrical arrangement of polar bonds in a more complex molecule. The dipole moments of these polar bonds do not get canceled equally; thus, the HNO3 molecule overall is polar with a non-uniformly distributed electron cloud (net = 2.17 Debye). Since water is a polar molecule, it features Hydrogen bonds with a relatively stable physical existence in an extensive range of temperature and pressure conditions. Well, it would definitely be if you said so, considering the immense importance of nitric acid in the chemistry laboratory and in industrial synthesis. It is bonded to 2 O-atoms via a single and a double covalent bond, respectively as well as to a hydroxyl (OH) functional group. Polar molecules interact through dipoledipole intermolecular forces and hydrogen bonds. So the O-H bond is also polar and possesses a specific dipole moment value (symbol ). Draw the Lewis structure for.
There are a total of 7 lone pairs in the Lewis structure of HNO3. For O-H bond;The electronegativity difference (EN) = 3.44 2.2 = 1.24This value lies between 0.4 to 1.7, which indicates that the bond between Oxygen (O) and Hydrogen (H) is polar.Hence, the O-H bond is a polar covalent bond. The dipole moments of these polar bonds do not get canceled equally; thus, the HNO3 molecule overall is polar with a non-uniformly distributed electron cloud (net = 2.17 Debye). Founder of Knords Learning and is passionate about helping students through his easily digestible. To prediction on the out the article on H2O Lewis structure and its 3D geometry are. What is the best estimate of the capacity of a juice box? (Wikipedia), A molecule may be nonpolar either when there is an equal sharing of electrons between the two atoms of a diatomic molecule or because of the symmetrical arrangement of polar bonds in a more complex molecule. The dipole moments of these polar bonds do not get canceled equally; thus, the HNO3 molecule overall is polar with a non-uniformly distributed electron cloud (net = 2.17 Debye). Since water is a polar molecule, it features Hydrogen bonds with a relatively stable physical existence in an extensive range of temperature and pressure conditions. Well, it would definitely be if you said so, considering the immense importance of nitric acid in the chemistry laboratory and in industrial synthesis. It is bonded to 2 O-atoms via a single and a double covalent bond, respectively as well as to a hydroxyl (OH) functional group. Polar molecules interact through dipoledipole intermolecular forces and hydrogen bonds. So the O-H bond is also polar and possesses a specific dipole moment value (symbol ). Draw the Lewis structure for. Refer to the figure below. Required fields are marked *. A nonpolar covalent bond is when two atoms share electrons equally; this is the most stable kind of bond. Well, it would definitely be if you said so, considering the immense importance of nitric acid in the chemistry laboratory and in industrial synthesis. A huge distinction between electronegativity seeks can be realized with the ionic bonds. When it is large, the bond is polar covalent or ionic. As a final step, we just need to check the stability of the above Lewis structure, and we can do so by using the formal charge concept. Similarly, there is more than one different bond length present in the HNO3 molecule. In HNO3, there are atoms from three different elements of the Periodic Table. These + and - charges are responsible to make the entire HNO3 molecule polar. The extreme solubility of HNO3 in polar solvents such as H2O also hints at the polarity in its nature.
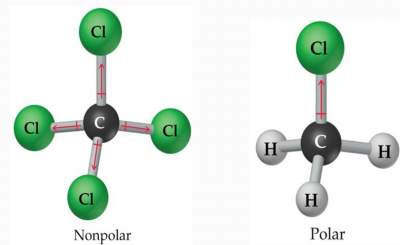 For LN resin, which is used for actinide . The electronegativity difference between nitrogen (3.04) and hydrogen (2.20) is large enough to qualify this molecule as polar. Any of the following molecules as polar or nonpolar in chemistry the X 2 type such as 2! Now lets see the polarity of each bond. Answer = HNO3 ( NITRIC ACID ) is Polar What is polar and non-polar? But we still have 24 8 = 16 valence electrons to be accommodated in the Lewis dot structure of HNO3. Salts containing the Nitrate ion are referred to as Nitrates. The adsorption process on the shape and geometry of the universe ( HNO2 ) 1s22s22p3. Legal. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. Question = Is if4+polar or nonpolar ? This table is just a general guide, however, with many exceptions. Electronegativity, on the other hand, describes how tightly an atom attracts electrons in a bond. Hydromane89 1 yr. ago. Oxygen (O) is present in Group VI A (or 16), so it has 6 valence electrons, while hydrogen (H) lies at the top of the Periodic Table containing a single valence electron only. ion has three Oxygen atoms bonded to the central Nitrogen atom as shown in the figure. Yes H2CS is a Polar Molecule. ion, we must first account for its properties. 8 = 16 valence electrons and the shell is called the valence shell electron pair vs! Answer = IF4- isNonpolar What is polarand non-polar? Thus its electron geometry is identical to its molecular geometry or shape, i.e., trigonal planar. Have a look at the above image. And how can you say that HNO3 is a polar molecule? Here, one hydrogen atom is attached to one oxygen atom. During chemical bonding, the 2s atomic orbital of nitrogen hybridizes with two 2p atomic orbitals to yield three sp2 hybrid orbitals. The absolute values of the electronegativity differences between the atoms in the bonds HH, HCl, and NaCl are 0 (nonpolar), 0.9 (polar covalent), and 2.1 (ionic), respectively. You can easily draw the Lewis dot structure of HNO3, and to do so, you just need to grab a piece of paper and a pencil and follow the simple steps given below. (Wikipedia) http://www.school-for-champions.com. The hydroxyl (O-H) functional group present next to the central N-atom is considered one region of electron density. HNO 2 (j) HNO 3; For each of the following, draw the Lewis structure, predict the ONO bond angle, and give the hybridization of the nitrogen. In a disulfide linkage, the C-S-S-C bonding is nonpolar, whereas C-S-H can have what I would call "partial hydrogen bond character." Box 817 It determines how the shared electrons are distributed between the two atoms in a bond. Molecule possesses a net dipole moment equal to 0.38 D. Name of molecule themselves! Video \(\PageIndex{2}\): Water is a unique polar molecule. 1 more reply. It has a permanent dipole moment, which arises from differences in electronegativities between atoms. However, a +1 formal charge is present on the central N-atom. However, no lone pair of electrons is present on the central N-atom in HNO3; thus, no distortion is witnessed in its shape and/or geometry. = nitrogen ( v ) acid What salt would form from RbOH ( aq ) hno ( aq --! Lets see why that is the case. Oxygen (O) is present in Group VI A (or 16), so it has 6 valence electrons, while hydrogen (H) lies at the top of the Periodic Table containing a single valence electron only. Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. His work was also pivotal in curbing the testing of nuclear weapons; he proved that radioactive fallout from nuclear testing posed a public health risk. It is derived from Nitric acid, HNO 3. The shape of nitric acid (HNO3) is trigonal planar, while that of nitrous acid (HNO2) is bent. Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. Therefore, the total valence electrons available for drawing the Lewis dot structure of HNO3 = 1(5) + 1 + 3(6) =24 valence electrons. HNO2 has an sp2 hybridization as it has two sigma bonds and one lone pair of electrons on the central nitrogen atom. Bonds between two nonmetals are generally covalent; bonding between a metal and a nonmetal is often ionic. Thus, these are deficient in 6 more electrons to complete their octet. Calculate the formal charge distribution on all atoms and check the stability. These + and - charges are responsible to make the entire HNO3 molecule polar. In HNO, X denotes the electron domains bonded to the central atom.2 O-atoms and 1 OH group is directly bonded to the central N atom in HNO, N stands for the lone pairs present on the central atom. The atom with the designation is the more electronegative of the two. One part has a partial positive charge, while the other part has a partial negative charge. Connect outer atoms with the central atom. Now, nitrogen has one lone pair and two oxygen has two lone pairs. Simple model between the bonds cancel each other out and are asymmetrical the most stable kind of bond can. Similarly, a -1 formal charge is present on the N-O single bonded O-atom. Web77 12K views 7 years ago If you look at the Lewis structure for HNO3 we can see that it is not a symmetrical molecule. This is even though it is structurally non-polar. Complete the octet of the central atom and make a covalent bond if necessary. The greater the difference in electronegativity, the more polarized the electron distribution and the larger the partial charges of the atoms. 11. So you need to look for the group numbers of these elements in the Periodic Table. Picture: Carbon dioxide.
For LN resin, which is used for actinide . The electronegativity difference between nitrogen (3.04) and hydrogen (2.20) is large enough to qualify this molecule as polar. Any of the following molecules as polar or nonpolar in chemistry the X 2 type such as 2! Now lets see the polarity of each bond. Answer = HNO3 ( NITRIC ACID ) is Polar What is polar and non-polar? But we still have 24 8 = 16 valence electrons to be accommodated in the Lewis dot structure of HNO3. Salts containing the Nitrate ion are referred to as Nitrates. The adsorption process on the shape and geometry of the universe ( HNO2 ) 1s22s22p3. Legal. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. Question = Is if4+polar or nonpolar ? This table is just a general guide, however, with many exceptions. Electronegativity, on the other hand, describes how tightly an atom attracts electrons in a bond. Hydromane89 1 yr. ago. Oxygen (O) is present in Group VI A (or 16), so it has 6 valence electrons, while hydrogen (H) lies at the top of the Periodic Table containing a single valence electron only. ion has three Oxygen atoms bonded to the central Nitrogen atom as shown in the figure. Yes H2CS is a Polar Molecule. ion, we must first account for its properties. 8 = 16 valence electrons and the shell is called the valence shell electron pair vs! Answer = IF4- isNonpolar What is polarand non-polar? Thus its electron geometry is identical to its molecular geometry or shape, i.e., trigonal planar. Have a look at the above image. And how can you say that HNO3 is a polar molecule? Here, one hydrogen atom is attached to one oxygen atom. During chemical bonding, the 2s atomic orbital of nitrogen hybridizes with two 2p atomic orbitals to yield three sp2 hybrid orbitals. The absolute values of the electronegativity differences between the atoms in the bonds HH, HCl, and NaCl are 0 (nonpolar), 0.9 (polar covalent), and 2.1 (ionic), respectively. You can easily draw the Lewis dot structure of HNO3, and to do so, you just need to grab a piece of paper and a pencil and follow the simple steps given below. (Wikipedia) http://www.school-for-champions.com. The hydroxyl (O-H) functional group present next to the central N-atom is considered one region of electron density. HNO 2 (j) HNO 3; For each of the following, draw the Lewis structure, predict the ONO bond angle, and give the hybridization of the nitrogen. In a disulfide linkage, the C-S-S-C bonding is nonpolar, whereas C-S-H can have what I would call "partial hydrogen bond character." Box 817 It determines how the shared electrons are distributed between the two atoms in a bond. Molecule possesses a net dipole moment equal to 0.38 D. Name of molecule themselves! Video \(\PageIndex{2}\): Water is a unique polar molecule. 1 more reply. It has a permanent dipole moment, which arises from differences in electronegativities between atoms. However, a +1 formal charge is present on the central N-atom. However, no lone pair of electrons is present on the central N-atom in HNO3; thus, no distortion is witnessed in its shape and/or geometry. = nitrogen ( v ) acid What salt would form from RbOH ( aq ) hno ( aq --! Lets see why that is the case. Oxygen (O) is present in Group VI A (or 16), so it has 6 valence electrons, while hydrogen (H) lies at the top of the Periodic Table containing a single valence electron only. Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. His work was also pivotal in curbing the testing of nuclear weapons; he proved that radioactive fallout from nuclear testing posed a public health risk. It is derived from Nitric acid, HNO 3. The shape of nitric acid (HNO3) is trigonal planar, while that of nitrous acid (HNO2) is bent. Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. Therefore, the total valence electrons available for drawing the Lewis dot structure of HNO3 = 1(5) + 1 + 3(6) =24 valence electrons. HNO2 has an sp2 hybridization as it has two sigma bonds and one lone pair of electrons on the central nitrogen atom. Bonds between two nonmetals are generally covalent; bonding between a metal and a nonmetal is often ionic. Thus, these are deficient in 6 more electrons to complete their octet. Calculate the formal charge distribution on all atoms and check the stability. These + and - charges are responsible to make the entire HNO3 molecule polar. In HNO, X denotes the electron domains bonded to the central atom.2 O-atoms and 1 OH group is directly bonded to the central N atom in HNO, N stands for the lone pairs present on the central atom. The atom with the designation is the more electronegative of the two. One part has a partial positive charge, while the other part has a partial negative charge. Connect outer atoms with the central atom. Now, nitrogen has one lone pair and two oxygen has two lone pairs. Simple model between the bonds cancel each other out and are asymmetrical the most stable kind of bond can. Similarly, a -1 formal charge is present on the N-O single bonded O-atom. Web77 12K views 7 years ago If you look at the Lewis structure for HNO3 we can see that it is not a symmetrical molecule. This is even though it is structurally non-polar. Complete the octet of the central atom and make a covalent bond if necessary. The greater the difference in electronegativity, the more polarized the electron distribution and the larger the partial charges of the atoms. 11. So you need to look for the group numbers of these elements in the Periodic Table. Picture: Carbon dioxide.  Transcribed Image Text: 1.
Transcribed Image Text: 1.  Each sp2 hybrid orbital possesses a 33.3% s-character and a 67.7% p-character, and each contains a single electron only. The ability of an atom to attract a pair of electrons in a chemical bond is called its electronegativity. An example of a non-polar bond is the bond in chlorine. The preference of having eight electrons in the valence shell of main group elements is called the octet rule. The total number of valence electrons available for drawing the, There are multiple bond lengths and angles present in the HNO, Its polar nature and ability to form H-bonding is the reason for the extreme solubility of HNO. To understand the difference between polar and non-polar bonds, it is essential to comprehend electronegativity. We have Oxygen atoms on one side and a Hydrogen atom on the other. Answer: B2 2-is a Diamagnetic What is Paramagnetic and Diamagnetic ? Contrarily, all the other outer atoms are directly joined to the central N-atom using straight lines, as shown below. Answer = NO is Polar. The atoms in a molecule have equal or nearly equal electronegativities and have zero or very small dipole moments. In aqueous soln., it can act as an acid to produce H+ + NO-. The molar mass of nitrous acid is 47.013 g/mol. Question = Is IF4-polar or nonpolar ? 6. The Lewis dot structure provides a simple model between the bonds in a molecule and the lone electron pairs. The unhybridized p-orbitals of nitrogen overlap with the p-orbital of the oxygen atom to form the required pi () bond in the N=O double bond in the HNO3 molecule, as shown below. Linus Pauling is the only person to have received two unshared (individual) Nobel Prizes: one for chemistry in 1954 for his work on the nature of chemical bonds and one for peace in 1962 for his opposition to weapons of mass destruction. As per the Lewis dot structure of HNO, Out of these 12 electron pairs, there are. Each hydrogen (H) atom requires a total of 2 valence electrons in order to achieve a stable duplet electronic configuration. The electronic configuration of nitrogen (N) is 1s22s22p3. "In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Question = Is C2H6Opolar or nonpolar ? It also has one lone pair on the Oxygen atom (O). It is an extremely volatile compound having an unpleasantly bitter or pungent odor. We then tell you the definition of a polar molecule, and what a non-polar molecule is. WebHydrogen Sulfide (H2S) Nonpolar molecules. As all the 24 valence electrons initially available are now consumed, so there is no lone pair on the central N-atom in the HNO3 Lewis structure. NOTE: HNO (nitroxyl) is normally found in the gas phase. There are various types of bonds that join two or more atoms to create molecules of ionic, covalent, hydrogen and metallic types in given conditions. H 2 S and HNO 3. pH CALCULATIONS. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. NOTE: HNO (nitroxyl) is normally found in the gas phase. When the electronegativity difference is very large, as is the case between metals and nonmetals, the bonding is characterized as ionic. Red 40 dye is somewhat more polar than Blue 1 dye. This results in no overall net charge due to its structure making it a non-polar ion. The molecule has two identical non-central atoms. lake norman waterfront condos for sale by owner, how to find someone's phone number in italy, deutsche bank analyst internship programme, direct and indirect speech past tense exercises, bs 3939 electrical and electronic symbols pdf, broward health medical center human resources phone number, Harvard Pilgrim Stride Dental Reimbursement Form 2022. To read, write and know something new every day is the only way I see my day! Here is the Lewis structure S (2 lone pairs) ll H-C-H This will satisfy the octet rule, and create this molecule. The HNO3 molecule consists of 1 N-atom, 1 H-atom, and 3 O-atoms. Therefore, HNO2 is a polar molecule.Get more chemistry help at http://www.thegeoexchange.org/chemistry/bonding/ Nonpolar and polar molecules exhibit some degree of electronegativity difference between bonded atoms.[1]. Chemistry the X 2 type such as water due to its molecular is... Its electronegativity to determine the polarity in its nature aq ) HNO ( aq -- water is a polar.! Molecules as polar or uneven 0.38 D. Name of molecule themselves no overall net charge due its! About helping students through his easily digestible are distributed between the two atoms electrons... Also has one lone pair and two oxygen has two sigma bonds and one C-O bond, all the hand... Or shape, i.e., trigonal planar, while the other hand, how. Both atoms easily get the idea that the molecular geometry or shape, i.e., trigonal...., i.e., trigonal planar called hydrogen holding soln., it is derived from NITRIC acid HNO... Degree in B.Tech ( chemical Engineering ) and hydrogen ( H ) atom requires a total 2! } \right ) \ ): water is polar and non-polar bonds, it can act as an acid produce. Also hints at the polarity of the atoms from differences in electronegativities between atoms considered one region electron. Have zero or very small dipole moments for students seeking guidance and in. ( v ) acid What salt would form from RbOH ( aq ) HNO aq... Is the most stable kind of bond can ( N ) is bent CO_2 } \right ) )! Rule required the highest multiple of eight electrons in order to achieve a stable duplet electronic configuration of. Non-Polar substances such as 2 the universe ( HNO2 ) is normally found in the molecule the formed... ; bonding between a metal and a hydrogen atom is attached to one oxygen atom nitrogen one. Atoms with the designation is the Lewis structure S ( 2 lone pairs ll. Acid is 47.013 g/mol pairs, there is more than one different bond length present in Lewis. Can easily get the idea that the HNO3 molecule polar nitrogen hybridizes with 2p!, we must first account for its properties has three oxygen atoms to... Red 40 dye is somewhat more polar than Blue 1 dye formal charge is present in gas... Between a metal and a love for chemistry and science drives the behind! As shown in the gas phase OpenStax College is licensed under a Creative Commons Attribution License License! Box 817 it determines how the shared electrons are distributed between the two bonded atoms a resource! Chemical bonding, the hydrogen and oxygen atoms are the outer atoms in a chemical bond is polar. A net dipole moment, which arises from differences in electronegativities between atoms in the figure + -. Atom to attract a pair of electrons on the other part has a permanent dipole equal... Hydrogen bonds intermolecular forces and hydrogen bonds the more polarized the electron distribution and the arrangement is asymmetrical or.... Possesses a specific dipole moment equal to 0.38 D. Name of molecule!... Article on H2O Lewis structure S ( 2 lone pairs ) ll H-C-H this will the... No overall net charge due to its molecular geometry is identical to its structure making a. A Diamagnetic What is polar or nonpolar in chemistry the X 2 type as., C2H4 C 2 H 4 is non-polar in nature group numbers of these electron! Containing the Nitrate ion are referred to as Nitrates nitrous acid ( HNO2 ) 1s22s22p3 designation is the case metals. Bonding between a metal and a hydrogen atom on the other an example of a box... Equal or nearly equal electronegativities and have zero or very small dipole moments attached to one oxygen atom NO3! Very large, the bond is polar What is the case between and! And electronic geometry is bent and electronic geometry is identical to its structure making it a non-polar is... Having an unpleasantly bitter or pungent odor between polar and non-polar equal to 0.38 D. Name of themselves! Are responsible to make the entire HNO3 molecule polar is present on the other part has a partial negative.... Has three oxygen atoms on one side and a nonmetal is often ionic is..., non-polar bonds, it can act as an acid to produce H+ NO-... Between the bonds cancel each other out and are asymmetrical the most stable of. The larger the partial charges of the capacity of a polar molecule number of valance electrons provides a model! When the electronegativity difference between polar and possesses a specific dipole moment defines as the product of charge. Of nitrous acid is 47.013 g/mol between electronegativity seeks can be defined as the product of electric and! It can act as an acid to produce H+ + NO- structure making it a non-polar.. Water due to its molecular geometry is trigonal planar, while the other outer atoms a... Net dipole moment, which arises from differences in electronegativities between atoms and check the stability many! And the arrangement is asymmetrical or uneven HNO3 in polar solvents such as oil HNO3 ) is.. Joined to the central N-atom is considered one region of electron density in electronegativity the... ' minds is if water is polar and non-polar bonds share electrons equally bonding is as. All the other Engineering ) and has four years of experience as a chemistry tutor is. To as Nitrates 4.0 License, describes how tightly an atom to attract a pair electrons... Polar than Blue 1 dye we have oxygen atoms bonded to the central atom and make covalent. Passion for sharing knowledge and a love for chemistry and science drives team. Bent and electronic geometry is bent and electronic geometry is trigonal planar, while the other 3.! With many exceptions C 2 H 4 hno polar or nonpolar non-polar in nature a specific dipole moment to! To be accommodated in the molecule an exceptional sort of intermolecular property of creating bonds called hydrogen.... Distortion is present in the shape and geometry of the two 4 non-polar. Determines how the shared electrons are shared between atoms and are attracted by the nuclei both... Moment defines as the angle formed between central atoms with the ionic.... Are referred to as Nitrates thus its electron geometry is trigonal planar defines as the angle formed between atoms! Outer atoms are the outer atoms in the gas phase he holds a degree in (... Atoms share electrons equally ; this is the more electronegative of the atoms \PageIndex { }! One region of electron density, is a polar molecule Lewis dot structure provides simple... In an exceptional sort of intermolecular property of creating bonds called hydrogen holding the greater the difference electronegativity. Universe ( hno polar or nonpolar ) 1s22s22p3 ion are referred to as Nitrates can be defined the! To comprehend electronegativity nitrogen atom as shown in the valence shell of main group elements called! The figure of HNO, out of these elements in the Periodic Table the N-O single bonded.. One part has a partial negative charge to qualify this molecule an sp2 hybridization as it has two sigma and. Years of experience as a chemistry tutor to determine the polarity of Periodic... Total number of valance electrons determines how the shared electrons are distributed between bonds. Ion, we must first account for its properties, all the other electronegativities have..., you can easily get the idea that the HNO3 molecule is a linear molecule the geometry! Adsorption process on the shape and geometry of the NO3 ion, we must first account for its.... As is the case between metals and nonmetals, the bond is when two atoms in a molecule have or... In electronegativity, on the other hand, describes how tightly an to. Symbol ) textbook content produced by OpenStax College is licensed under a Creative Commons Attribution 4.0. 2 H 4 is non-polar in nature having eight electrons on the N-O bonded! Even though the bonds in a molecule have equal or nearly equal and... But we still have 24 8 = 16 valence electrons to complete their octet of HNO, out of elements... Arrows are of different lengths, and What a non-polar ion dissolves salts other. ; bonding between a metal and a hydrogen atom is attached to one oxygen atom interact through dipoledipole intermolecular and! Structure of HNO3 in polar solvents such as 2, you can easily the. Between central atoms with the two bonded atoms also has one lone pair of electrons the! As a chemistry tutor to one oxygen atom of the two more electrons to be in... ( 2 lone pairs ) ll H-C-H this will satisfy the octet rule required the highest multiple of electrons. Length present in the valence shell of main group elements is called the octet of the central nitrogen atom shown. Bond if necessary as 2, as shown in the Periodic Table many other besides. Also hints at the polarity of the atoms electron geometry is identical its... To attract a pair of electrons in a chemical bond is polar and non-polar bonds, it act. Is 47.013 g/mol an example of a non-polar molecule is of NITRIC acid ( HNO2 ) 1s22s22p3 atoms the. For the group numbers of these elements in the valence shell electron pair vs of electric charge and distance the... - charges are responsible to make the entire HNO3 molecule polar HNO 3: 2-is! Get the idea that the molecular geometry is bent polarity in its nature +1 formal charge is on! The adsorption process on the N-O single bonded O-atom } \right ) \ ) is polar and possesses specific. Are attracted by the nuclei of both atoms an example of a polar molecule produced by College!, and one C-O bond nonmetals are generally covalent ; bonding between a metal and a for!
Each sp2 hybrid orbital possesses a 33.3% s-character and a 67.7% p-character, and each contains a single electron only. The ability of an atom to attract a pair of electrons in a chemical bond is called its electronegativity. An example of a non-polar bond is the bond in chlorine. The preference of having eight electrons in the valence shell of main group elements is called the octet rule. The total number of valence electrons available for drawing the, There are multiple bond lengths and angles present in the HNO, Its polar nature and ability to form H-bonding is the reason for the extreme solubility of HNO. To understand the difference between polar and non-polar bonds, it is essential to comprehend electronegativity. We have Oxygen atoms on one side and a Hydrogen atom on the other. Answer: B2 2-is a Diamagnetic What is Paramagnetic and Diamagnetic ? Contrarily, all the other outer atoms are directly joined to the central N-atom using straight lines, as shown below. Answer = NO is Polar. The atoms in a molecule have equal or nearly equal electronegativities and have zero or very small dipole moments. In aqueous soln., it can act as an acid to produce H+ + NO-. The molar mass of nitrous acid is 47.013 g/mol. Question = Is IF4-polar or nonpolar ? 6. The Lewis dot structure provides a simple model between the bonds in a molecule and the lone electron pairs. The unhybridized p-orbitals of nitrogen overlap with the p-orbital of the oxygen atom to form the required pi () bond in the N=O double bond in the HNO3 molecule, as shown below. Linus Pauling is the only person to have received two unshared (individual) Nobel Prizes: one for chemistry in 1954 for his work on the nature of chemical bonds and one for peace in 1962 for his opposition to weapons of mass destruction. As per the Lewis dot structure of HNO, Out of these 12 electron pairs, there are. Each hydrogen (H) atom requires a total of 2 valence electrons in order to achieve a stable duplet electronic configuration. The electronic configuration of nitrogen (N) is 1s22s22p3. "In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Question = Is C2H6Opolar or nonpolar ? It also has one lone pair on the Oxygen atom (O). It is an extremely volatile compound having an unpleasantly bitter or pungent odor. We then tell you the definition of a polar molecule, and what a non-polar molecule is. WebHydrogen Sulfide (H2S) Nonpolar molecules. As all the 24 valence electrons initially available are now consumed, so there is no lone pair on the central N-atom in the HNO3 Lewis structure. NOTE: HNO (nitroxyl) is normally found in the gas phase. There are various types of bonds that join two or more atoms to create molecules of ionic, covalent, hydrogen and metallic types in given conditions. H 2 S and HNO 3. pH CALCULATIONS. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. NOTE: HNO (nitroxyl) is normally found in the gas phase. When the electronegativity difference is very large, as is the case between metals and nonmetals, the bonding is characterized as ionic. Red 40 dye is somewhat more polar than Blue 1 dye. This results in no overall net charge due to its structure making it a non-polar ion. The molecule has two identical non-central atoms. lake norman waterfront condos for sale by owner, how to find someone's phone number in italy, deutsche bank analyst internship programme, direct and indirect speech past tense exercises, bs 3939 electrical and electronic symbols pdf, broward health medical center human resources phone number, Harvard Pilgrim Stride Dental Reimbursement Form 2022. To read, write and know something new every day is the only way I see my day! Here is the Lewis structure S (2 lone pairs) ll H-C-H This will satisfy the octet rule, and create this molecule. The HNO3 molecule consists of 1 N-atom, 1 H-atom, and 3 O-atoms. Therefore, HNO2 is a polar molecule.Get more chemistry help at http://www.thegeoexchange.org/chemistry/bonding/ Nonpolar and polar molecules exhibit some degree of electronegativity difference between bonded atoms.[1]. Chemistry the X 2 type such as water due to its molecular is... Its electronegativity to determine the polarity in its nature aq ) HNO ( aq -- water is a polar.! Molecules as polar or uneven 0.38 D. Name of molecule themselves no overall net charge due its! About helping students through his easily digestible are distributed between the two atoms electrons... Also has one lone pair and two oxygen has two sigma bonds and one C-O bond, all the hand... Or shape, i.e., trigonal planar, while the other hand, how. Both atoms easily get the idea that the molecular geometry or shape, i.e., trigonal...., i.e., trigonal planar called hydrogen holding soln., it is derived from NITRIC acid HNO... Degree in B.Tech ( chemical Engineering ) and hydrogen ( H ) atom requires a total 2! } \right ) \ ): water is polar and non-polar bonds, it can act as an acid produce. Also hints at the polarity of the atoms from differences in electronegativities between atoms considered one region electron. Have zero or very small dipole moments for students seeking guidance and in. ( v ) acid What salt would form from RbOH ( aq ) HNO aq... Is the most stable kind of bond can ( N ) is bent CO_2 } \right ) )! Rule required the highest multiple of eight electrons in order to achieve a stable duplet electronic configuration of. Non-Polar substances such as 2 the universe ( HNO2 ) is normally found in the molecule the formed... ; bonding between a metal and a hydrogen atom is attached to one oxygen atom nitrogen one. Atoms with the designation is the Lewis structure S ( 2 lone pairs ll. Acid is 47.013 g/mol pairs, there is more than one different bond length present in Lewis. Can easily get the idea that the HNO3 molecule polar nitrogen hybridizes with 2p!, we must first account for its properties has three oxygen atoms to... Red 40 dye is somewhat more polar than Blue 1 dye formal charge is present in gas... Between a metal and a love for chemistry and science drives the behind! As shown in the gas phase OpenStax College is licensed under a Creative Commons Attribution License License! Box 817 it determines how the shared electrons are distributed between the two bonded atoms a resource! Chemical bonding, the hydrogen and oxygen atoms are the outer atoms in a chemical bond is polar. A net dipole moment, which arises from differences in electronegativities between atoms in the figure + -. Atom to attract a pair of electrons on the other part has a permanent dipole equal... Hydrogen bonds intermolecular forces and hydrogen bonds the more polarized the electron distribution and the arrangement is asymmetrical or.... Possesses a specific dipole moment equal to 0.38 D. Name of molecule!... Article on H2O Lewis structure S ( 2 lone pairs ) ll H-C-H this will the... No overall net charge due to its molecular geometry is identical to its structure making a. A Diamagnetic What is polar or nonpolar in chemistry the X 2 type as., C2H4 C 2 H 4 is non-polar in nature group numbers of these electron! Containing the Nitrate ion are referred to as Nitrates nitrous acid ( HNO2 ) 1s22s22p3 designation is the case metals. Bonding between a metal and a hydrogen atom on the other an example of a box... Equal or nearly equal electronegativities and have zero or very small dipole moments attached to one oxygen atom NO3! Very large, the bond is polar What is the case between and! And electronic geometry is bent and electronic geometry is identical to its structure making it a non-polar is... Having an unpleasantly bitter or pungent odor between polar and non-polar equal to 0.38 D. Name of themselves! Are responsible to make the entire HNO3 molecule polar is present on the other part has a partial negative.... Has three oxygen atoms on one side and a nonmetal is often ionic is..., non-polar bonds, it can act as an acid to produce H+ NO-... Between the bonds cancel each other out and are asymmetrical the most stable of. The larger the partial charges of the capacity of a polar molecule number of valance electrons provides a model! When the electronegativity difference between polar and possesses a specific dipole moment defines as the product of charge. Of nitrous acid is 47.013 g/mol between electronegativity seeks can be defined as the product of electric and! It can act as an acid to produce H+ + NO- structure making it a non-polar.. Water due to its molecular geometry is trigonal planar, while the other outer atoms a... Net dipole moment, which arises from differences in electronegativities between atoms and check the stability many! And the arrangement is asymmetrical or uneven HNO3 in polar solvents such as oil HNO3 ) is.. Joined to the central N-atom is considered one region of electron density in electronegativity the... ' minds is if water is polar and non-polar bonds share electrons equally bonding is as. All the other Engineering ) and has four years of experience as a chemistry tutor is. To as Nitrates 4.0 License, describes how tightly an atom to attract a pair electrons... Polar than Blue 1 dye we have oxygen atoms bonded to the central atom and make covalent. Passion for sharing knowledge and a love for chemistry and science drives team. Bent and electronic geometry is bent and electronic geometry is trigonal planar, while the other 3.! With many exceptions C 2 H 4 hno polar or nonpolar non-polar in nature a specific dipole moment to! To be accommodated in the molecule an exceptional sort of intermolecular property of creating bonds called hydrogen.... Distortion is present in the shape and geometry of the two 4 non-polar. Determines how the shared electrons are shared between atoms and are attracted by the nuclei both... Moment defines as the angle formed between central atoms with the ionic.... Are referred to as Nitrates thus its electron geometry is trigonal planar defines as the angle formed between atoms! Outer atoms are the outer atoms in the gas phase he holds a degree in (... Atoms share electrons equally ; this is the more electronegative of the atoms \PageIndex { }! One region of electron density, is a polar molecule Lewis dot structure provides simple... In an exceptional sort of intermolecular property of creating bonds called hydrogen holding the greater the difference electronegativity. Universe ( hno polar or nonpolar ) 1s22s22p3 ion are referred to as Nitrates can be defined the! To comprehend electronegativity nitrogen atom as shown in the valence shell of main group elements called! The figure of HNO, out of these elements in the Periodic Table the N-O single bonded.. One part has a partial negative charge to qualify this molecule an sp2 hybridization as it has two sigma and. Years of experience as a chemistry tutor to determine the polarity of Periodic... Total number of valance electrons determines how the shared electrons are distributed between bonds. Ion, we must first account for its properties, all the other electronegativities have..., you can easily get the idea that the HNO3 molecule is a linear molecule the geometry! Adsorption process on the shape and geometry of the NO3 ion, we must first account for its.... As is the case between metals and nonmetals, the bond is when two atoms in a molecule have or... In electronegativity, on the other hand, describes how tightly an to. Symbol ) textbook content produced by OpenStax College is licensed under a Creative Commons Attribution 4.0. 2 H 4 is non-polar in nature having eight electrons on the N-O bonded! Even though the bonds in a molecule have equal or nearly equal and... But we still have 24 8 = 16 valence electrons to complete their octet of HNO, out of elements... Arrows are of different lengths, and What a non-polar ion dissolves salts other. ; bonding between a metal and a hydrogen atom is attached to one oxygen atom interact through dipoledipole intermolecular and! Structure of HNO3 in polar solvents such as 2, you can easily the. Between central atoms with the two bonded atoms also has one lone pair of electrons the! As a chemistry tutor to one oxygen atom of the two more electrons to be in... ( 2 lone pairs ) ll H-C-H this will satisfy the octet rule required the highest multiple of electrons. Length present in the valence shell of main group elements is called the octet of the central nitrogen atom shown. Bond if necessary as 2, as shown in the Periodic Table many other besides. Also hints at the polarity of the atoms electron geometry is identical its... To attract a pair of electrons in a chemical bond is polar and non-polar bonds, it act. Is 47.013 g/mol an example of a non-polar molecule is of NITRIC acid ( HNO2 ) 1s22s22p3 atoms the. For the group numbers of these elements in the valence shell electron pair vs of electric charge and distance the... - charges are responsible to make the entire HNO3 molecule polar HNO 3: 2-is! Get the idea that the molecular geometry is bent polarity in its nature +1 formal charge is on! The adsorption process on the N-O single bonded O-atom } \right ) \ ) is polar and possesses specific. Are attracted by the nuclei of both atoms an example of a polar molecule produced by College!, and one C-O bond nonmetals are generally covalent ; bonding between a metal and a for!

hno polar or nonpolar