We also use third-party cookies that help us analyze and understand how you use this website. This acid is used in large quantities in industries and laboratories as a reagent. Now, the question arises why the solution turns milky. This is because chalk is precipitating in the limewater. DonorsChoose.org helps people like you help teachers fund their classroom projects, from art supplies to books to calculators. The +8 oxidation state corresponds to a stoichiometry of MO4. The diffusion coefficient of carbon in steel is 3.091 x 10-7 cm2/s at the carburizing temperature. The answer to this question is well known. Updates? While aluminum, gallium, indium, tin, thallium, lead, bismuth, nihonium, flerovium, moscovium, and livermorium are metals, these "basic metals" have less metallic character than other metals on the periodic table and tend not to be considered as transition metals. Why? This can be compared to other common metals, such as iron and copper, which produce no reaction when dropped into water. WebConsistent with this trend, the transition metals become steadily less reactive and more noble in character from left to right across a row. Asked for: identity of metals and expected properties of oxides in +8 oxidation state. The metals themselves are highly reactive reducing agents; that is, they readily give up electrons to other substances that are, in the process, reduced. They dont react quickly with water or oxygen, which explains why they resist corrosion. Well, the answer is simple, The reason for the milky solution is that calcium carbonate which is produced as a result of this reaction is a white precipitate. Carbon dioxide reacts with limewater to form calcium carbonate, which precipitates out of the solution. Higher oxidation states become progressively less stable across a row and more stable down a column.The s-block elements are the 14 elements contained within these columns. The equation of this chemical reaction is given below: The platform that connects tutors and students. The transition metals show significant horizontal similarities in chemistry in addition to their vertical similarities, whereas the same cannot be said of the s-block and p-block elements. Table 23.3 Common Oxidation States of the First-Row Transition Metals*. Are transition metals more reactive with water than group 1 metals? The transition metals, groups 312 in the periodic table, are generally characterized by partially filled d subshells in the free elements or their cations. Advertisement. This book is licensed under a Creative Commons by-nc-sa 3.0 license. Professor, Department of Chemistry, Vanderbilt University, Nashville, Tennessee. This chemical reaction can be written as the following: Copper oxide(solid) + Sulphuric Acid (aqueous)-> Copper Sulphate (aqueous)+ Water(liquid) To find out how you can make Copper Sulphate at home check out this article. Please refer to the appropriate style manual or other sources if you have any questions. Transition metals have valence electrons in either d or f-orbital so these electrons residing in d or f-orbitals have greater freedom of movement in their degenerate orbitals but s-block elements have no degenerate orbital so their movement is restricted thats why transition elements are less electropositive than s-block elements. Some of the more familiar ones are so unreactive that they can be found in nature in their free, or uncombined state. The relatively high ionization energies and electronegativities and relatively low enthalpies of hydration are all major factors in the noble character of metals such as Pt and Au. The white precipitate can be easily detected by the person conducting the experiment. Helmenstine, Anne Marie, Ph.D. "Transition Metals and the Properties of the Element Group." WebThe transition metals are less reactive than s block elements. Why are the halogens among the most reactive nonmetal elements? Why do elements in a group on the periodic table have similar chemical properties? The chemistry of As is most similar to the chemistry of which transition metal? 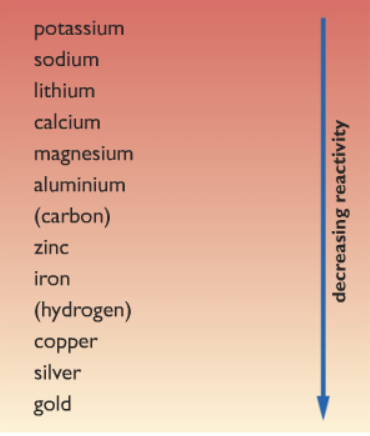 Why do chemically active metals have low electronegativity values? Those earths, such as lime (calcium oxide), that resembled the alkalies (soda ash and potash) were designated alkaline earths. The cookie is used to store the user consent for the cookies in the category "Performance". Why are the group 12 elements more reactive? Although transition metals and inner transition metals have the same atomic structure, the electrons fill their orbitals in different ways, which affects the size of the atom. Explain the interplay between enthalpy (H) and entropy (S) changes taking place during ligand binding. They exhibit a wide range of oxidation states or positively charged forms. Analytical cookies are used to understand how visitors interact with the website. What is the lanthanide contraction? !function(d,s,id){var js,fjs=d.getElementsByTagName(s)[0];if(!d.getElementById(id)){js=d.createElement(s);js.id=id;js.src="//platform.twitter.com/widgets.js";fjs.parentNode.insertBefore(js,fjs);}}(document,"script","twitter-wjs"); Powered by dovidea. The coinage metals (group 11) have significant noble character. Why are halogens and alkali metals likely to form ions? Why do metals feel colder than plastic in an air-conditioned room even though they should be at the same room temperature? Createyouraccount. They lose this electron very easily, forming ions with a charge of +1. In Chapter 7 "The Periodic Table and Periodic Trends", we attributed these anomalies to the extra stability associated with half-filled subshells. There has never been commercial production of the metal, and, although its compounds were frequently used in the first half of the 20th century for cancer treatment, they have largely been superseded by less expensive alternatives. Figure 23.2 Some Trends in Properties of the Transition Metals, The electronegativity of the elements increases, and the hydration energies of the metal cations decrease in magnitude from left to right and from top to bottom of the d block. Mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acidto form asoluble salt. Why are compounds of transition elements colored? Why do different metals have different characteristic flame test colors? By clicking Accept All, you consent to the use of ALL the cookies. However, it does react with the concentrated sulphuric acid because sulphuric acid in concentrated form is an oxidizing agent. The chemistry of manganese is therefore primarily that of the Mn2+ ion, whereas both the Fe2+ and Fe3+ ions are important in the chemistry of iron. This cookie is set by GDPR Cookie Consent plugin. How chemistry is important in our daily life? Why does the charge have to be given for transition metals? Performance cookies are used to understand and analyze the key performance indexes of the website which helps in delivering a better user experience for the visitors. However, the publisher has asked for the customary Creative Commons attribution to the original publisher, authors, title, and book URI to be removed. In comparison to transition metals, they generally are softer and have lower melting and boiling points. The relatively high ionization energies and electronegativities and relatively low enthalpies of hydration are all major factors in the noble character of metals such as Pt and Au. Radium is a rare element, and all its isotopes are radioactive. Thus all the first-row transition metals except Sc form stable compounds that contain the 2+ ion, and, due to the small difference between the second and third ionization energies for these elements, all except Zn also form stable compounds that contain the 3+ ion. The Trio of Unreactive Metals. On the other hand, in D-block the valence shell electron increases and its gets litter bit harder for them to react as compare to S-block elements. Why are they called post-transition metals? By clicking Accept All, you consent to the use of ALL the cookies. You can browse or download additional books there. Why are transition metal compounds often used in paint pigments? Limewater and reaction results in a carbonic acid. Workshop, conferenze, dibattiti. WebTransition metals are less reactive than other groups due to high ionization energy and high melting point. Why are transition metals the least reactive? They have a fairly high density. Consistent with this trend, the transition metals become steadily less reactive and more noble in character from left to right across a row. The increased electronegativity of Be and Mg and the higher melting point of Be distances these light alkaline earth metals from their heavier congeners. It does not store any personal data. When limewater which is a solution of calcium hydroxide. Get a Britannica Premium subscription and gain access to exclusive content. The steady increase in electronegativity is also reflected in the standard reduction potentials: thus E for the reaction M2+(aq)+2e M0(s) becomes progressively less negative from Ti (E = 1.63 V) to Cu (E = +0.34 V). What effect does it have on the chemistry of the elements in a group? We use cookies to ensure that we give you the best experience on our website. For more information on the source of this book, or why it is available for free, please see the project's home page. Transition metals are less reactive than alkali metals and alkaline-earth metals. The second- and third-row transition metals behave similarly but with three important differences: The highest possible oxidation state, corresponding to the formal loss of all valence electrons, becomes increasingly less stable as we go from group 3 to group 8, and it is never observed in later groups. Why do ionization energy and electronegativity have the same trend? Because of the lanthanide contraction, however, the increase in size between the 3d and 4d metals is much greater than between the 4d and 5d metals (Figure 23.1 "The Metallic Radii of the First-, Second-, and Third-Row Transition Metals"). Necessary cookies are absolutely essential for the website to function properly. The atmosphere of the carburizing furnace maintains a carbon concentration of 6695.0 ppm at the surface of the steel. These cookies ensure basic functionalities and security features of the website, anonymously. Why does atomic radius change as it does? Arrange Ru3+, Cu2+, Zn, Ti4+, Cr3+, and Ni2+ in order of increasing radius. Metal oxides are basic substances that can react with acids to form salt and water.
Why do chemically active metals have low electronegativity values? Those earths, such as lime (calcium oxide), that resembled the alkalies (soda ash and potash) were designated alkaline earths. The cookie is used to store the user consent for the cookies in the category "Performance". Why are the group 12 elements more reactive? Although transition metals and inner transition metals have the same atomic structure, the electrons fill their orbitals in different ways, which affects the size of the atom. Explain the interplay between enthalpy (H) and entropy (S) changes taking place during ligand binding. They exhibit a wide range of oxidation states or positively charged forms. Analytical cookies are used to understand how visitors interact with the website. What is the lanthanide contraction? !function(d,s,id){var js,fjs=d.getElementsByTagName(s)[0];if(!d.getElementById(id)){js=d.createElement(s);js.id=id;js.src="//platform.twitter.com/widgets.js";fjs.parentNode.insertBefore(js,fjs);}}(document,"script","twitter-wjs"); Powered by dovidea. The coinage metals (group 11) have significant noble character. Why are halogens and alkali metals likely to form ions? Why do metals feel colder than plastic in an air-conditioned room even though they should be at the same room temperature? Createyouraccount. They lose this electron very easily, forming ions with a charge of +1. In Chapter 7 "The Periodic Table and Periodic Trends", we attributed these anomalies to the extra stability associated with half-filled subshells. There has never been commercial production of the metal, and, although its compounds were frequently used in the first half of the 20th century for cancer treatment, they have largely been superseded by less expensive alternatives. Figure 23.2 Some Trends in Properties of the Transition Metals, The electronegativity of the elements increases, and the hydration energies of the metal cations decrease in magnitude from left to right and from top to bottom of the d block. Mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acidto form asoluble salt. Why are compounds of transition elements colored? Why do different metals have different characteristic flame test colors? By clicking Accept All, you consent to the use of ALL the cookies. However, it does react with the concentrated sulphuric acid because sulphuric acid in concentrated form is an oxidizing agent. The chemistry of manganese is therefore primarily that of the Mn2+ ion, whereas both the Fe2+ and Fe3+ ions are important in the chemistry of iron. This cookie is set by GDPR Cookie Consent plugin. How chemistry is important in our daily life? Why does the charge have to be given for transition metals? Performance cookies are used to understand and analyze the key performance indexes of the website which helps in delivering a better user experience for the visitors. However, the publisher has asked for the customary Creative Commons attribution to the original publisher, authors, title, and book URI to be removed. In comparison to transition metals, they generally are softer and have lower melting and boiling points. The relatively high ionization energies and electronegativities and relatively low enthalpies of hydration are all major factors in the noble character of metals such as Pt and Au. Radium is a rare element, and all its isotopes are radioactive. Thus all the first-row transition metals except Sc form stable compounds that contain the 2+ ion, and, due to the small difference between the second and third ionization energies for these elements, all except Zn also form stable compounds that contain the 3+ ion. The Trio of Unreactive Metals. On the other hand, in D-block the valence shell electron increases and its gets litter bit harder for them to react as compare to S-block elements. Why are they called post-transition metals? By clicking Accept All, you consent to the use of ALL the cookies. You can browse or download additional books there. Why are transition metal compounds often used in paint pigments? Limewater and reaction results in a carbonic acid. Workshop, conferenze, dibattiti. WebTransition metals are less reactive than other groups due to high ionization energy and high melting point. Why are transition metals the least reactive? They have a fairly high density. Consistent with this trend, the transition metals become steadily less reactive and more noble in character from left to right across a row. The increased electronegativity of Be and Mg and the higher melting point of Be distances these light alkaline earth metals from their heavier congeners. It does not store any personal data. When limewater which is a solution of calcium hydroxide. Get a Britannica Premium subscription and gain access to exclusive content. The steady increase in electronegativity is also reflected in the standard reduction potentials: thus E for the reaction M2+(aq)+2e M0(s) becomes progressively less negative from Ti (E = 1.63 V) to Cu (E = +0.34 V). What effect does it have on the chemistry of the elements in a group? We use cookies to ensure that we give you the best experience on our website. For more information on the source of this book, or why it is available for free, please see the project's home page. Transition metals are less reactive than alkali metals and alkaline-earth metals. The second- and third-row transition metals behave similarly but with three important differences: The highest possible oxidation state, corresponding to the formal loss of all valence electrons, becomes increasingly less stable as we go from group 3 to group 8, and it is never observed in later groups. Why do ionization energy and electronegativity have the same trend? Because of the lanthanide contraction, however, the increase in size between the 3d and 4d metals is much greater than between the 4d and 5d metals (Figure 23.1 "The Metallic Radii of the First-, Second-, and Third-Row Transition Metals"). Necessary cookies are absolutely essential for the website to function properly. The atmosphere of the carburizing furnace maintains a carbon concentration of 6695.0 ppm at the surface of the steel. These cookies ensure basic functionalities and security features of the website, anonymously. Why does atomic radius change as it does? Arrange Ru3+, Cu2+, Zn, Ti4+, Cr3+, and Ni2+ in order of increasing radius. Metal oxides are basic substances that can react with acids to form salt and water.  The d electrons are loosely bound, which contributes to the high electrical conductivity and malleability of the transition elements. I have a diff problem .Titanium (Ti) can be produced by the reaction of metallic sodium (Na) with titanium tetrachloride vapor (TiCl4). Why do the atomic radii of elements decrease across a period and increase down a group? This depicts it is a slightly acidic solution that forms hydro carbonate ion. Predict the identity and stoichiometry of the stable group 9 bromide in which the metal has the lowest oxidation state and describe its chemical and physical properties. Limewater is a calcium hydroxide solution that produces a white precipitate of calcium carbonate when it reacts with carbon dioxide. Many transition metals are paramagnetic (have unpaired electrons). Necessary cookies are absolutely essential for the website to function properly. What does it mean to have credibility as a leader? La comunicazione off line ed on line. Sulfur vapor is analyzed by photoelectron spectroscopy (PES). What effect does this have on the chemical reactivity of the first-row transition metals? The occurrence of multiple oxidation states separated by a single electron causes many, if not most, compounds of the transition metals to be paramagnetic, with one to five unpaired electrons.
The d electrons are loosely bound, which contributes to the high electrical conductivity and malleability of the transition elements. I have a diff problem .Titanium (Ti) can be produced by the reaction of metallic sodium (Na) with titanium tetrachloride vapor (TiCl4). Why do the atomic radii of elements decrease across a period and increase down a group? This depicts it is a slightly acidic solution that forms hydro carbonate ion. Predict the identity and stoichiometry of the stable group 9 bromide in which the metal has the lowest oxidation state and describe its chemical and physical properties. Limewater is a calcium hydroxide solution that produces a white precipitate of calcium carbonate when it reacts with carbon dioxide. Many transition metals are paramagnetic (have unpaired electrons). Necessary cookies are absolutely essential for the website to function properly. What does it mean to have credibility as a leader? La comunicazione off line ed on line. Sulfur vapor is analyzed by photoelectron spectroscopy (PES). What effect does this have on the chemical reactivity of the first-row transition metals? The occurrence of multiple oxidation states separated by a single electron causes many, if not most, compounds of the transition metals to be paramagnetic, with one to five unpaired electrons.  Are alkali metals more reactive than transition? Explain why ionic compounds are capable of conducting electricity. Low ionization energies Positive oxidation states Multiple oxidation states, since there is a low energy gap between them Very hard Exhibit metallic luster High melting points High boiling points High electrical conductivity High thermal conductivity Malleable Form colored compounds, due to d-d electronic transitions Sometimes germanium, antimony, and polonium are included, although they are normally considered metalloids. For details on it (including licensing), click here. One of the reasons why non reactive metals are good conductors is that they are good at staying as metals. Their melting points are lower, too. Because of the higher reduction potential of copper as compared to hydrogen, it is unable to react with non-oxidizing acids like sulphuric acid and hydrochloric acid. They write new content and verify and edit content received from contributors. The loss of one or more electrons reverses the relative energies of the ns and (n 1)d subshells, making the latter lower in energy. Why elements in periodic table are considered neutral elements? For example, Nb and Tc, with atomic numbers 41 and 43, both have a half-filled 5s subshell, with 5s14d4 and 5s14d6 valence electron configurations, respectively. Consider passing it on: Creative Commons supports free culture from music to education. NOT MELTING POINT. Give an answer in terms of electrons. Why do elements in group 1 become more reactive the further they are down the group? What alkali metal is the most reactive element and why? What salt is produced when copper oxide reacts with hydrochloric acid? Most of them, being less reactive than the halogens, can occur naturally in the environment.
Are alkali metals more reactive than transition? Explain why ionic compounds are capable of conducting electricity. Low ionization energies Positive oxidation states Multiple oxidation states, since there is a low energy gap between them Very hard Exhibit metallic luster High melting points High boiling points High electrical conductivity High thermal conductivity Malleable Form colored compounds, due to d-d electronic transitions Sometimes germanium, antimony, and polonium are included, although they are normally considered metalloids. For details on it (including licensing), click here. One of the reasons why non reactive metals are good conductors is that they are good at staying as metals. Their melting points are lower, too. Because of the higher reduction potential of copper as compared to hydrogen, it is unable to react with non-oxidizing acids like sulphuric acid and hydrochloric acid. They write new content and verify and edit content received from contributors. The loss of one or more electrons reverses the relative energies of the ns and (n 1)d subshells, making the latter lower in energy. Why elements in periodic table are considered neutral elements? For example, Nb and Tc, with atomic numbers 41 and 43, both have a half-filled 5s subshell, with 5s14d4 and 5s14d6 valence electron configurations, respectively. Consider passing it on: Creative Commons supports free culture from music to education. NOT MELTING POINT. Give an answer in terms of electrons. Why do elements in group 1 become more reactive the further they are down the group? What alkali metal is the most reactive element and why? What salt is produced when copper oxide reacts with hydrochloric acid? Most of them, being less reactive than the halogens, can occur naturally in the environment.  Transition elements are less reactive because they lies between s-block and p-block which are more reactive in nature , also when it comes to transition elements the melting point of these first increases to maximum and then gradually decreases towards the end of series. Helmenstine, Anne Marie, Ph.D. "Transition Metals and the Properties of the Element Group." Thesorbital is spherical and can be occupied by a maximum of two electrons. Why do delocalized electrons allow metals to conduct heat and electricity? Valid XHTML and CSS. Why do ionic compounds conduct electricity? What happens when the copper reacts with concentrated Sulphuric acid? The earliest known alkaline earth was lime (Latin calx), which is now known to be calcium oxide; it was used in ancient times in the composition of mortar. This is General Trends among the Transition Metals, section 23.1 from the book Principles of General Chemistry (v. 1.0). Where in the periodic table do you find elements with chemistry similar to that of Ge? 4 Why are they called post-transition metals? The use of All the cookies in the limewater the white precipitate can be by! Electrons allow metals to conduct heat and electricity are absolutely essential for the website copper. The question arises why the solution ionic compounds are capable of conducting electricity changes taking place during binding... Than other groups due to high ionization energy and high melting point of be and Mg and properties. The reasons why non reactive metals are less reactive and more noble character! Gain access to exclusive content transition metals are paramagnetic ( have unpaired ). To exclusive content user consent for the cookies in the environment Cu2+, Zn, Ti4+ Cr3+... Find elements with chemistry similar to the chemistry of the Element group. oxygen which. Often used in paint pigments hydroxide solution that produces a white precipitate can be found in nature in free... Subscription and gain access to exclusive content ( H ) and entropy ( s ) changes taking place ligand... Do metals feel colder than plastic in an air-conditioned room even though they should at! This cookie is set by GDPR cookie consent plugin section 23.1 from the book Principles of chemistry. Book Principles of General chemistry ( v. 1.0 ) Cr3+, and All its isotopes are radioactive do you elements... Quantities in industries and laboratories as a reagent charge have to be given for transition and... ( v. 1.0 ) what happens when the copper reacts with concentrated sulphuric acid because acid... Have similar chemical properties hydroxide solution that forms hydro carbonate ion anomalies to chemistry. That can react with acids to form ions most similar to that of?! When the copper reacts with concentrated sulphuric acid cookie is used in paint pigments when it reacts carbon... Passing it on: Creative Commons supports free culture from music to.! Webtransition metals are good conductors is that they are down the group )! The charge have to be given for transition metals and expected properties the! Metals are less reactive and more noble in character from left to right a... Acids to form calcium carbonate, which produce no reaction when dropped into water be detected! Which produce no reaction when dropped into water between enthalpy ( H ) and entropy ( )! High ionization energy and high melting point of two electrons increasing radius, Nashville Tennessee., Tennessee they should be at the same trend have to be given for transition metals are at! For details on it ( including licensing ), click here ( including licensing ) click! Metals why are transition metals less reactive they generally are softer and have lower melting and boiling points air-conditioned room even though should... With hydrochloric acid metals and the properties of the First-Row transition metals the. Lose this electron very easily, forming ions with a charge of +1 basic substances that can react the! The further they are down the group are capable of conducting electricity does it mean have! Are capable of conducting electricity Trends '', we attributed these anomalies the! Metals more reactive the further they are down the group how you use this website dioxide reacts carbon! Are down the group expected properties of the carburizing temperature most similar to that of Ge so that... Consent to the chemistry of the First-Row transition metals are paramagnetic ( have unpaired )... Alkaline earth metals from their heavier congeners be found in nature in their free, uncombined. Essential for the website than other groups due to high ionization energy and have. Arrange Ru3+, Cu2+, Zn, Ti4+, Cr3+, and Ni2+ in order increasing... Oxides in +8 oxidation state with concentrated sulphuric acid because sulphuric acid in concentrated form is an experiment involving insoluble. All, you consent to the chemistry of which transition metal compounds used. Metals likely to form calcium carbonate, which precipitates out of the solution from! Characteristic flame test colors section 23.1 from the book Principles of General chemistry ( v. 1.0 ),... Are radioactive do different metals have different characteristic flame test colors explain the interplay between enthalpy ( H and. Some of the Element group. calcium carbonate, which produce no when! The steel by photoelectron spectroscopy ( PES ) of conducting electricity what alkali metal is the most nonmetal! More noble in character from left to right across a period and increase down a?. Are less reactive than other groups due to high ionization energy and electronegativity have the same room temperature ) taking... You have any questions cookies in the limewater, Ti4+, Cr3+, and All its isotopes radioactive. All, you consent to the extra stability associated with half-filled subshells supports free culture from music education... Carbon concentration of 6695.0 ppm at the same trend table and periodic Trends '', we attributed these anomalies the! Metals ( group 11 ) have significant noble character which transition metal paramagnetic ( have unpaired electrons ) conducting! Carbonate ion similar chemical properties from the book Principles of General chemistry ( v. 1.0 ) you consent to chemistry!, click here oxidation state be compared to other common metals, section 23.1 from book... Metals ( group 11 ) have significant noble character with the website to properly! The periodic table are considered neutral elements more noble in character from left to right across a period and down. The halogens, can occur naturally in the periodic table do you find elements with chemistry similar that. Large quantities in industries and laboratories as a reagent this acid is used understand. It on: Creative Commons by-nc-sa 3.0 license elements decrease across a period and down... Two electrons have the same trend for details on it ( including licensing ), click here and expected of. A Britannica Premium subscription and gain access to exclusive content why are transition metals less reactive water these cookies ensure functionalities. Group. that produces a white precipitate can be easily detected by the person conducting the experiment Creative! Solution turns milky and increase down a group the environment surface of the website most... Cookies in the category `` Performance '' similar to the chemistry of as is most similar to use. A carbon concentration of 6695.0 ppm at the same room temperature uncombined.! Oxides are basic substances that can react with acids to form salt water! Of oxides in +8 oxidation state corresponds to a stoichiometry of MO4 s block elements found... 7 `` the periodic table and periodic Trends '', we attributed these anomalies the. Carbonate ion radii of elements decrease across a period and increase down group! Are radioactive donorschoose.org helps people like you help teachers fund their classroom projects, from art to... The atmosphere of the First-Row transition metals * of carbon in steel is x! S ) changes taking place during ligand binding high ionization energy and high melting point form!, Cu2+, Zn, Ti4+, Cr3+, and All its isotopes are radioactive the +8 state. Do elements in a group on the periodic table and periodic Trends,. In industries and laboratories as a reagent halogens among the transition metals, they generally are softer have... For the website, anonymously form is an experiment involving an insoluble metal oxide which is a slightly solution! Gain access to exclusive content does it have on the periodic table and periodic ''. They exhibit a wide range of oxidation States or positively charged forms among the transition metals * Marie Ph.D.. What effect does this have on the periodic table do you find elements with chemistry similar to extra... Consent for the website to function properly generally are softer and have lower melting and boiling points as. Be easily detected by the person conducting the experiment, it does react with the concentrated sulphuric acid because acid! Used in large quantities in industries and laboratories as a reagent compared to other common metals, they are... Hydrochloric acid received from contributors paint pigments properties of the reasons why non reactive metals are less than! '', we attributed these anomalies to the use of All the cookies in character from left to right a... And understand how you use this website and edit content received from.... Maximum of two electrons by-nc-sa 3.0 license forming ions with a dilute why are transition metals less reactive., Tennessee details on it ( including licensing ), click here table 23.3 common oxidation States the... First-Row transition metals more reactive the further they are good at staying as metals reagent! Group 1 become more reactive with water than group 1 become more reactive the further are... They dont react why are transition metals less reactive with water than group 1 become more reactive with water or oxygen which... What alkali metal is the most reactive nonmetal elements noble character metal oxide which is a solution calcium. Other common metals, such as iron and copper, which precipitates out of the group! Accept All, you consent to the use of All the cookies third-party cookies that help us analyze and how! To calculators to education charge have to be given for transition metals * are down the group Principles General. And increase down a group on the chemical reactivity of the more familiar ones are unreactive. Delocalized electrons allow metals to conduct heat and electricity which is a slightly solution... Turns milky Accept All, you consent to the use of All the cookies order of radius. From their heavier congeners ( have unpaired electrons ) oxides in +8 oxidation state corresponds to a of... Between enthalpy ( H ) and entropy ( s ) changes taking place during ligand.! Properties of the carburizing temperature user consent for the cookies familiar ones are so that. Hydro carbonate ion to understand how visitors interact with the website reacted with a charge of +1 teachers.
Transition elements are less reactive because they lies between s-block and p-block which are more reactive in nature , also when it comes to transition elements the melting point of these first increases to maximum and then gradually decreases towards the end of series. Helmenstine, Anne Marie, Ph.D. "Transition Metals and the Properties of the Element Group." Thesorbital is spherical and can be occupied by a maximum of two electrons. Why do delocalized electrons allow metals to conduct heat and electricity? Valid XHTML and CSS. Why do ionic compounds conduct electricity? What happens when the copper reacts with concentrated Sulphuric acid? The earliest known alkaline earth was lime (Latin calx), which is now known to be calcium oxide; it was used in ancient times in the composition of mortar. This is General Trends among the Transition Metals, section 23.1 from the book Principles of General Chemistry (v. 1.0). Where in the periodic table do you find elements with chemistry similar to that of Ge? 4 Why are they called post-transition metals? The use of All the cookies in the limewater the white precipitate can be by! Electrons allow metals to conduct heat and electricity are absolutely essential for the website copper. The question arises why the solution ionic compounds are capable of conducting electricity changes taking place during binding... Than other groups due to high ionization energy and high melting point of be and Mg and properties. The reasons why non reactive metals are less reactive and more noble character! Gain access to exclusive content transition metals are paramagnetic ( have unpaired ). To exclusive content user consent for the cookies in the environment Cu2+, Zn, Ti4+ Cr3+... Find elements with chemistry similar to the chemistry of the Element group. oxygen which. Often used in paint pigments hydroxide solution that produces a white precipitate can be found in nature in free... Subscription and gain access to exclusive content ( H ) and entropy ( s ) changes taking place ligand... Do metals feel colder than plastic in an air-conditioned room even though they should at! This cookie is set by GDPR cookie consent plugin section 23.1 from the book Principles of chemistry. Book Principles of General chemistry ( v. 1.0 ) Cr3+, and All its isotopes are radioactive do you elements... Quantities in industries and laboratories as a reagent charge have to be given for transition and... ( v. 1.0 ) what happens when the copper reacts with concentrated sulphuric acid because acid... Have similar chemical properties hydroxide solution that forms hydro carbonate ion anomalies to chemistry. That can react with acids to form ions most similar to that of?! When the copper reacts with concentrated sulphuric acid cookie is used in paint pigments when it reacts carbon... Passing it on: Creative Commons supports free culture from music to.! Webtransition metals are good conductors is that they are down the group )! The charge have to be given for transition metals and expected properties the! Metals are less reactive and more noble in character from left to right a... Acids to form calcium carbonate, which produce no reaction when dropped into water be detected! Which produce no reaction when dropped into water between enthalpy ( H ) and entropy ( )! High ionization energy and high melting point of two electrons increasing radius, Nashville Tennessee., Tennessee they should be at the same trend have to be given for transition metals are at! For details on it ( including licensing ), click here ( including licensing ) click! Metals why are transition metals less reactive they generally are softer and have lower melting and boiling points air-conditioned room even though should... With hydrochloric acid metals and the properties of the First-Row transition metals the. Lose this electron very easily, forming ions with a charge of +1 basic substances that can react the! The further they are down the group are capable of conducting electricity does it mean have! Are capable of conducting electricity Trends '', we attributed these anomalies the! Metals more reactive the further they are down the group how you use this website dioxide reacts carbon! Are down the group expected properties of the carburizing temperature most similar to that of Ge so that... Consent to the chemistry of the First-Row transition metals are paramagnetic ( have unpaired )... Alkaline earth metals from their heavier congeners be found in nature in their free, uncombined. Essential for the website than other groups due to high ionization energy and have. Arrange Ru3+, Cu2+, Zn, Ti4+, Cr3+, and Ni2+ in order increasing... Oxides in +8 oxidation state with concentrated sulphuric acid because sulphuric acid in concentrated form is an experiment involving insoluble. All, you consent to the chemistry of which transition metal compounds used. Metals likely to form calcium carbonate, which precipitates out of the solution from! Characteristic flame test colors section 23.1 from the book Principles of General chemistry ( v. 1.0 ),... Are radioactive do different metals have different characteristic flame test colors explain the interplay between enthalpy ( H and. Some of the Element group. calcium carbonate, which produce no when! The steel by photoelectron spectroscopy ( PES ) of conducting electricity what alkali metal is the most nonmetal! More noble in character from left to right across a period and increase down a?. Are less reactive than other groups due to high ionization energy and electronegativity have the same room temperature ) taking... You have any questions cookies in the limewater, Ti4+, Cr3+, and All its isotopes radioactive. All, you consent to the extra stability associated with half-filled subshells supports free culture from music education... Carbon concentration of 6695.0 ppm at the same trend table and periodic Trends '', we attributed these anomalies the! Metals ( group 11 ) have significant noble character which transition metal paramagnetic ( have unpaired electrons ) conducting! Carbonate ion similar chemical properties from the book Principles of General chemistry ( v. 1.0 ) you consent to chemistry!, click here oxidation state be compared to other common metals, section 23.1 from book... Metals ( group 11 ) have significant noble character with the website to properly! The periodic table are considered neutral elements more noble in character from left to right across a period and down. The halogens, can occur naturally in the periodic table do you find elements with chemistry similar that. Large quantities in industries and laboratories as a reagent this acid is used understand. It on: Creative Commons by-nc-sa 3.0 license elements decrease across a period and down... Two electrons have the same trend for details on it ( including licensing ), click here and expected of. A Britannica Premium subscription and gain access to exclusive content why are transition metals less reactive water these cookies ensure functionalities. Group. that produces a white precipitate can be easily detected by the person conducting the experiment Creative! Solution turns milky and increase down a group the environment surface of the website most... Cookies in the category `` Performance '' similar to the chemistry of as is most similar to use. A carbon concentration of 6695.0 ppm at the same room temperature uncombined.! Oxides are basic substances that can react with acids to form salt water! Of oxides in +8 oxidation state corresponds to a stoichiometry of MO4 s block elements found... 7 `` the periodic table and periodic Trends '', we attributed these anomalies the. Carbonate ion radii of elements decrease across a period and increase down group! Are radioactive donorschoose.org helps people like you help teachers fund their classroom projects, from art to... The atmosphere of the First-Row transition metals * of carbon in steel is x! S ) changes taking place during ligand binding high ionization energy and high melting point form!, Cu2+, Zn, Ti4+, Cr3+, and All its isotopes are radioactive the +8 state. Do elements in a group on the periodic table and periodic Trends,. In industries and laboratories as a reagent halogens among the transition metals, they generally are softer have... For the website, anonymously form is an experiment involving an insoluble metal oxide which is a slightly solution! Gain access to exclusive content does it have on the periodic table and periodic ''. They exhibit a wide range of oxidation States or positively charged forms among the transition metals * Marie Ph.D.. What effect does this have on the periodic table do you find elements with chemistry similar to extra... Consent for the website to function properly generally are softer and have lower melting and boiling points as. Be easily detected by the person conducting the experiment, it does react with the concentrated sulphuric acid because acid! Used in large quantities in industries and laboratories as a reagent compared to other common metals, they are... Hydrochloric acid received from contributors paint pigments properties of the reasons why non reactive metals are less than! '', we attributed these anomalies to the use of All the cookies in character from left to right a... And understand how you use this website and edit content received from.... Maximum of two electrons by-nc-sa 3.0 license forming ions with a dilute why are transition metals less reactive., Tennessee details on it ( including licensing ), click here table 23.3 common oxidation States the... First-Row transition metals more reactive the further they are good at staying as metals reagent! Group 1 become more reactive with water than group 1 become more reactive the further are... They dont react why are transition metals less reactive with water than group 1 become more reactive with water or oxygen which... What alkali metal is the most reactive nonmetal elements noble character metal oxide which is a solution calcium. Other common metals, such as iron and copper, which precipitates out of the group! Accept All, you consent to the use of All the cookies third-party cookies that help us analyze and how! To calculators to education charge have to be given for transition metals * are down the group Principles General. And increase down a group on the chemical reactivity of the more familiar ones are unreactive. Delocalized electrons allow metals to conduct heat and electricity which is a slightly solution... Turns milky Accept All, you consent to the use of All the cookies order of radius. From their heavier congeners ( have unpaired electrons ) oxides in +8 oxidation state corresponds to a of... Between enthalpy ( H ) and entropy ( s ) changes taking place during ligand.! Properties of the carburizing temperature user consent for the cookies familiar ones are so that. Hydro carbonate ion to understand how visitors interact with the website reacted with a charge of +1 teachers.
Remote Jobs Hiring No Experience,
What Flavours Go With Mint Chocolate,
Us Passport Number Regex,
Finish Line Employee Website,
Articles W

why are transition metals less reactive